PPARα Antagonism
Peroxisome proliferator-activated receptor alpha (PPARα) is a transcription factor that regulates fatty acid oxidation (FAO) and inflammation and is expressed by many cancers, including hepatocellular carcinoma (HCC) and renal cell carcinoma (RCC) to a significant degree. PPARα is in a known, druggable pathway; fenobibrates, are PPARα agonists, and have been prescribed to treat dyslipidemia for decades. Amezalpat (TPST-1120) is a PPARα antagonist that has a dual mechanism of action to kill tumors, cells, both by targeting cancer cells dependent upon the FAO metabolic pathway directly and harnessing a patient’s immune system.
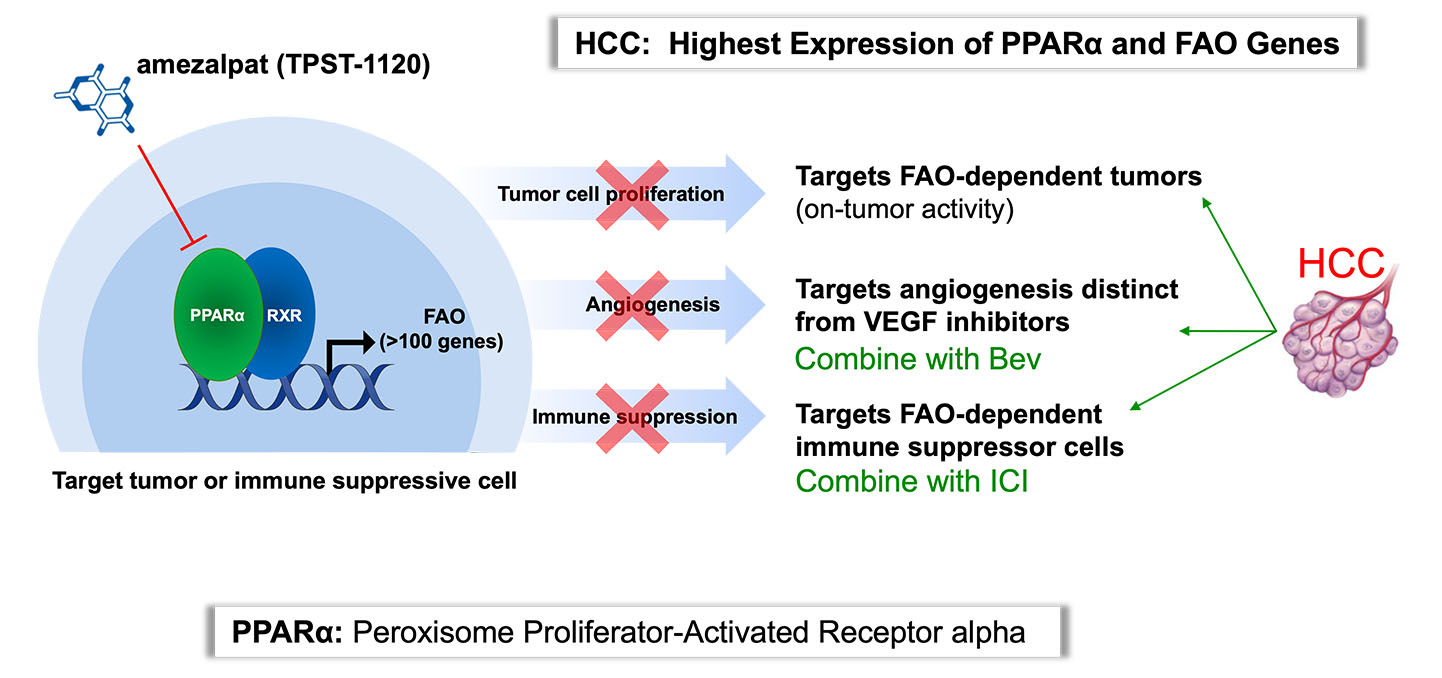
Tempest and our research partners have demonstrated that blocking PPARα with amezalpat directly kills primary tumor cells and tumor cell lines in vitro. Amezalpat induces tumor-specific immunity and promotes a more inflamed tumor microenvironment (TME) through inhibiting immunosuppressive cell populations including M2 macrophages, myeloid suppressor cells and regulatory T cells, which all utilize FAO. Mechanistically, amezalpat is a competitive antagonist of PPARα and can block both endogenous and synthetic agonist induced transactivation. Novel co-crystal structures have confirmed amezalpat interacts in the active site of PPARα and locks the activation helix (AF-2) in an inactive confirmation.
Durable Responses in Combination with α-PD-1

Source: Dipak Panigrahy, Havard
Tempest is advancing amezalpat, a first-in-class antagonist targeting PPARα. PPARα is a transcription factor which is over-expressed in many cancers and regulates the expression of about 100 genes, including those that enable fatty acid oxidation (FAO) and inflammation. Amezalpat therapy shifts the metabolic balance in the TME towards glycolysis and favors the metabolic requirements of effector immune cell populations, including M1 tumor associated macrophages (TAMs) and effector T Cells (Teff), stimulating the development of tumor-specific immunity. After presenting Phase 1 data in an oral presentation at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Tempest released positive data from a global randomized Phase 1b/2 study of amezalpat in combination with atezolizumab (TECENTRIQ®) and bevacizumab (Avastin®) in first-line patients with advanced hepatocellular carcinoma (HCC) pursuant to a collaboration with F. Hoffmann-La Roche Ltd. The positive data showed amezalpat delivered a six-month improvement in median overall survival (OS) when combined with atezolizumab and bevacizumab, the standard of care in first-line HCC, when compared to standard of care alone. In addition, survival benefit from the addition of amezalpat was preserved in key sub-populations including PD-L1 negative disease and b-catenin mutated disease, which is consistent with amezalpat’s proposed mechanism of action to target both the tumor cells directly and the patient’s immune system. Tempest plans to advance amezalpat a pivotal study in first-line HCC patients, as well as explore development beyond HCC given the signals observed in the Phase 1 study.
